Abstract
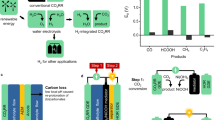
Supplying global energy demand with CO2-free technologies is becoming feasible thanks to the rising affordability of renewable resources. Hydrogen is a promising vector in the decarbonization of energy systems, but more efficient and scalable synthesis is required to enable its widespread deployment. Here we report contactless H2 production via water electrolysis mediated by the microwave-triggered redox activation of solid-state ionic materials at low temperatures (<250 °C). Water was reduced via reaction with non-equilibrium gadolinium-doped CeO2 that was previously in situ electrochemically deoxygenated by the sole application of microwaves. The microwave-driven reduction was identified by an instantaneous electrical conductivity rise and O2 release. This process was cyclable, whereas H2 yield and energy efficiency were material- and power-dependent. Deoxygenation of low-energy molecules (H2O or CO2) led to the formation of energy carriers and enabled CH4 production when integrated with a Sabatier reactor. This method could be extended to other reactions such as intensified hydrocarbons synthesis or oxidation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data used in this study are presented in the text, Supplementary Information and Source Data. Additional data and information are available from the corresponding author on request. Source data are provided with this paper.
References
Serra, J. M. Electrifying chemistry with protonic cells. Nat. Energy 4, 178–179 (2019).
Wei, M., McMillan, C. A. & de la Rue du Can, S. Electrification of industry: potential, challenges and outlook. Curr. Sustain. Energy Rep. 6, 140–148 (2019).
Malerød-Fjeld, H. et al. Thermo-electrochemical production of compressed hydrogen from methane with near-zero energy loss. Nat. Energy 2, 923–931 (2017).
Schiffer, Z. J. & Manthiram, K. Electrification and decarbonization of the chemical industry. Joule 1, 10–14 (2017).
Ran, J., Zhang, J., Yu, J., Jaroniec, M. & Qiao, S. Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 43, 7787–7812 (2014).
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
Vøllestad, E. et al. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 18, 752–759 (2019).
Duan, C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019).
Service, R. F. New electrolyzer splits water on the cheap. Science 367, 1181 (2020).
Hamzehlouia, S., Jaffer, S. A. & Chaouki, J. Microwave heating-assisted catalytic dry reforming of methane to syngas. Sci. Rep. 8, 8940 (2018).
Tsukahara, Y. et al. In situ observation of nonequilibrium local heating as an origin of special effect of microwave on chemistry. J. Phys. Chem. C 114, 8965–8970 (2010).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Eigen, J. & Schroeder, M. Redox cycling stability of Fe2NiO4/YSZ composite storage materials for rechargeable oxide batteries. Energy Storage Mater. 28, 112–121 (2020).
Catalá-Civera, J. M. et al. Dynamic measurement of dielectric properties of materials at high temperature during microwave heating in a dual mode cylindrical cavity. IEEE Trans. Microw. Theory Tech. 63, 2905–2914 (2012).
García-Baños, B., Reinosa, J. J., Peñaranda-Foix, F. L., Fernández, J. F. & Catalá-Civera, J. M. Temperature assessment of microwave-enhanced heating processes. Sci. Rep. 9, 10809 (2019).
Campbell, C. T. & Peden, C. H. F. Oxygen vacancies and catalysis on ceria surfaces. Science 309, 713–714 (2005).
Naghavi, S. S. et al. Giant onsite electronic entropy enhances the performance of ceria for water splitting. Nat. Commun. 8, 1–6 (2017).
Chueh, W. C. et al. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Sci. 330, 1797–1801 (2010).
Bulfin, B. et al. Analytical model of CeO2 oxidation and reduction. J. Phys. Chem. C 117, 24129–24137 (2013).
Geller, A. et al. Operando tracking of electrochemical activity in solid oxide electrochemical cells by using near-infrared imaging. ChemElectroChem 2, 1527–1534 (2015).
Balaguer, M., Solís, C. & Serra, J. M. Structural-transport properties relationships on Ce1–xLnxO2–δ system (Ln = Gd, La, Tb, Pr, Eu, Er, Yb, Nd) and effect of cobalt addition. J. Phys. Chem. C 116, 7975–7982 (2012).
Holstein, T. Studies of polaron motion. Part II. The ‘small’ polaron. Ann. Phys. (N. Y). 8, 343–389 (1959).
Seki, K. & Tachiya, M. Electric field dependence of charge mobility in energetically disordered materials: polaron aspects. Phys. Rev. B 65, 1–13 (2002).
Emin, D. Generalized adiabatic polaron hopping: Meyer–Neldel compensation and Poole–Frenkel behavior. Phys. Rev. Lett. 100, 166602 (2008).
Bishop, S. R., Duncan, K. L. & Wachsman, E. D. Surface and bulk oxygen non-stoichiometry and bulk chemical expansion in gadolinium-doped cerium oxide. Acta Mater. 57, 3596–3605 (2009).
Suzuki, T., Kosacki, I. & Anderson, H. U. Defect and mixed conductivity in nanocrystalline doped cerium oxide. J. Am. Ceram. Soc. 85, 1492–1498 (2002).
Zeng, L., Cheng, Z., Fan, J. A., Fan, L. S. & Gong, J. Metal oxide redox chemistry for chemical looping processes. Nat. Rev. Chem. 2, 349–364 (2018).
Liu, W., Song, M.-S., Kong, B. & Cui, Y. Flexible and stretchable energy storage: recent advances and future perspectives. Adv. Mater. 29, 1603436 (2017).
Berger, C. M. et al. Development of storage materials for high-temperature rechargeable oxide batteries. J. Energy Storage 1, 54–64 (2015).
Posdziech, O., Schwarze, K. & Brabandt, J. Efficient hydrogen production for industry and electricity storage via high-temperature electrolysis. Int. J. Hydrog. Energy 44, 19089–19101 (2019).
Maric, R. & Yu, H. In Nanostructures in Energy Generation, Transmission and Storage (IntechOpen, 2018).
Dincer, I. & Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 40, 11094–11111 (2015).
Gielen, D. Hydrogen from Renewable Power Technology Outlook for the Energy Transition (2018).
Kuckshinrichs, W., Ketelaer, T. & Koj, J. C. Economic analysis of improved alkaline water electrolysis. Front. Energy Res. 5, 1 (2017).
Holladay, J. D., Hu, J., King, D. L. & Wang, Y. An overview of hydrogen production technologies. Catal. Today 139, 244–260 (2009).
Glenk, G. & Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 4, 216–222 (2019).
Marxer, D., Furler, P., Takacs, M. & Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 10, 1142–1149 (2017).
Vogt, C., Monai, M., Kramer, G. J. & Weckhuysen, B. M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2, 188–197 (2019).
Eckle, S., Anfang, H. G. & Behm, R. J. Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases. J. Phys. Chem. C 115, 1361–1367 (2011).
Wei, Y. et al. Three-dimensionally ordered macroporous Ce0.8Zr0.2O2-supported gold nanoparticles: synthesis with controllable size and super-catalytic performance for soot oxidation. Energy Environ. Sci. 4, 2959–2970 (2011).
Santos, V. P., Pereira, M. F. R., Órfão, J. J. M. & Figueiredo, J. L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B 99, 353–363 (2010).
Hickman, D. A. & Schmidt, L. D. Production of syngas by direct catalytic oxidation of methane. Science 259, 343–346 (1993).
Feng, Z. A. et al. Fast vacancy-mediated oxygen ion incorporation across the ceria-gas electrochemical interface. Nat. Commun. 5, 1–9 (2014).
Flytzani-Stephanopoulos, M., Sakbodin, M. & Wang, Z. Regenerative adsorption and removal of H2S from hot fuel gas streams by rare earth oxides. Science 312, 1508–1510 (2006).
Paunović, V. et al. Europium oxybromide catalysts for efficient bromine looping in natural gas valorization. Angew. Chem. Int. Ed. 56, 9791–9795 (2017).
Krupka, J. Contactless methods of conductivity and sheet resistance measurement for semiconductors, conductors and superconductors. Meas. Sci. Technol. 24, 62001 (2013).
Altschuler, H. M. Handbook of Microwave Measurements Vol. 2 (Polytechnic Institute Brooklyn Press, 1963).
Arai, M., Binner, J. G. P. & Cross, T. E. Comparison of techniques for measuring high-temperature microwave complex permittivity: measurements on an alumina/zircona system. J. Microw. Power Electromagn. Energy 31, 12–18 (1996).
López, R. & Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: a comparative study. J. Sol.-Gel Sci. Technol. 61, 1–7 (2012).
Murphy, A. B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 91, 1326–1337 (2007).
Skorodumova, N. V. et al. Electronic, bonding, and optical properties of CeO2 and Ce2O3 from first principles. Phys. Rev. B 64, 1151081–1151089 (2001).
Acknowledgements
This work was supported by the Spanish Government (RTI2018-102161, SEV-2016-0683 and Juan de la Cierva grant IJCI-2017-34110). We thank the support of the Electronic Microscopy Service of the Universitat Politècnica de València.
Author information
Authors and Affiliations
Contributions
J.F.B.-M, P.P.-G., L.N and B.G.-B. performed the experiments. J.M.S., J.M.C.-C., M.B. and B.G.-B. designed the experiments. J.M.C.-C. and P.P.-G designed and fabricated the microwave-cavity assembly. J.F.B.-M, P.P.-G., B.G.-B., J.S.-B., and J.M.C.-C. analysed electrochemical and physical data. M.B, L.N., J.F.B.-M. and J.M.S performed the gas analyses and evaluated catalytic data. D.C.-M. performed thermodynamic and process simulations. M.B. collected physicochemical characterization. J.M.S. and J.M.C.-C. initiated the project. J.M.S., J.M.C.-C., L.N., J.S.-B., D.C.-M. and M.B. wrote the manuscript, whereas all authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The Universitat Politècnica de València and Consejo Superior de Investigaciones Científicas have jointly applied for a patent based on the method for microwave-driven reduction of oxides and its uses. The inventors are J.M.S., J.F.B.-M., B.G.-B., J.M.C.-C. and L.N and the Spanish priority number is ES2726028-B2.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, Notes 1–14, Tables 1–8 and references.
Source data
Source Data Fig. 2
Source data for plots b–d in Fig. 2. Figure 2b displays the flow of oxygen released, in ml min−1, and the CGO material temperature, in °C, as a function of time, in min. Figure 2c compares the electric conductivity behaviour of the solid-state material, in S m−1, for microwave-assisted and conventional heating as a function of inverse temperature, in K−1. Figure 2d reflects an analogous comparison for microwave-assisted heating above and below the material power threshold.
Source Data Fig. 3
Source data for plots b–d in Fig. 3. Figure 3b represents the time sequence, in s, of the control parameters for the microwave-assisted redox process that follows: material absorbed power, in W; temperature, in °C; electric conductivity, in S m−1; and H2 and O2 flows, in ml min−1. Figure 3c compares the time evolution, in s, of the microwave-assisted redox process with H2O and D2O, attending to the following control parameters: temperature, in °C; electric conductivity, in S m−1; and H2 and O2 flows, in arbitrary units. Figure 3d shows the time sequence, in s, of H2 and O2 flows, in ml min−1, and temperature, in °C, for several redox cycles.
Source Data Fig. 6
Source data for plots b and d in Fig. 6. Figure 6b displays the time evolution, in s, of the gas flows involved in CH4 production integrated with a Sabatier reaction, in ml min−1, that is O2, H2, CH4 and CO2. Figure 6d reflects analogous time sequence for the conversion of CH4 into syngas, which involves the gas flows for O2, H2, CH4 and CO, in ml min−1.
Rights and permissions
About this article
Cite this article
Serra, J.M., Borrás-Morell, J.F., García-Baños, B. et al. Hydrogen production via microwave-induced water splitting at low temperature. Nat Energy 5, 910–919 (2020). https://doi.org/10.1038/s41560-020-00720-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-00720-6
This article is cited by
-
External field assisted hydrogen evolution reaction
Nano Research (2023)
-
Selective catalytic oxidation of ammonia to nitric oxide via chemical looping
Nature Communications (2022)
-
Microwave-assisted molybdenum-nickel alloy for efficient water electrolysis under large current density through spillover and Fe doping
Nano Research (2022)
-
A mini review on microwave and contemporary based biohydrogen production technologies: a comparison
Environmental Science and Pollution Research (2022)
-
Cool water splitting by microwaves
Nature Energy (2020)